Introduction
Many states are prioritizing and, in some cases, requiring COVID-19 testing of all residents and staff at long-term care (LTC) facilities. This brief provides considerations for developing a strategy for COVID-19 testing in LTC facilities and a review of state actions to date. Additional details on testing and other state strategies for LTC facilities, including specific state resources can be found here. For the purposes of this brief, LTC facilities include nursing facilities (including post-acute), assisted living, and other residential care settings and testing approaches may vary across these settings.
Considerations for COVID-19 Testing in LTC Facilities
States may consider the following factors in developing a strategy for COVID-19 testing in LTC facilities:
(1) Types of tests and specimen collection methods
Diagnostic tests (such as polymerase chain reaction or antigen tests) detect infection and are critical to understanding day-to-day prevalence of COVID-19 and controlling spread within a facility. Reliability and quick results are particularly important in these settings. Balancing accuracy and rapid findings is key and requires thoughtful selection of tests and well-designed testing protocols. Rapid point of care testing (POC) is optimal for speed and tests should be selected based on accuracy data or partnership with clinical laboratories to develop protocols for rapid processing of lab-based tests. Effective specimen collection processes (e.g. nasal swab, saliva) that are collected or monitored by clinical staff are equally important. Serologic tests that detect virus antibodies may also be useful in informing infection control strategies in LTC facilities by providing greater understanding of past infections and future risks. Louisiana is an example of a state that is conducting serology testing in LTC facilities as part of its broader statewide testing approach. Just as with diagnostic tests, selecting serological tests for their accuracy profiles is important. The Food and Drug Administration maintains lists of approved tests, including review of serology test performance.
(2) Frequency for retesting residents and staff
Many states are taking steps to conduct baseline or initial testing of all residents and staff in LTC facilities, however, there is broad understanding that initial testing provides only a point in time assessment of COVID-19 prevalence. Consistent retesting, particularly of staff that exit and reenter the facilities regularly, is critical to identifying infections and preventing outbreaks on an ongoing basis. The Centers for Disease Control and Prevention and Centers for Medicare and Medicaid Services (CMS) have released guidance for retesting of residents and staff. As part of recommended reopening processes, the agencies recommend initial testing of all residents and staff and weekly testing of staff thereafter. In the case of an outbreak, facilities should test all residents and staff and repeat testing of previously negative individuals every 3 to 7 days until there are no new cases for at least 14 days. A number of states have begun requiring or recommending retesting in LTC facilities (additional details below).
(3) Access to supplies and staff capacity
For LTC facilities to successfully test residents and staff, it is imperative that they have adequate access to test kits and supplies, personal protective equipment and laboratory capacity to process results in a timely manner. It is also important that facilities have sufficient staff capacity not only to conduct testing but also to fill critical roles if more widespread testing reveals a significant number of cases among staff that would then be required to quarantine for at least 14 days. Workforce capacity in LTC facilities has already been a challenge and new testing requirements may elevate concerns around how to fill gaps and ensure quality patient care. States have taken a number of steps to address workforce issues, including supporting new and expanded roles such as temporary nurse aides and personal care attendants, increasing payments and providing other incentives for staff, and developing portals or other processes to connect facilities in need of staff with qualified individuals looking for work, among other approaches.
(4) Payment for tests and financial sustainability
Most LTC facilities residents are covered by Medicare or Medicaid and the cost of COVID-19 testing is reimbursable under both programs when ordered by an attending health care provider. However, there are challenges related to payment for testing of staff in LTC facilities, many of whom lack health coverage altogether or have a plan that does not cover surveillance testing. According to recent guidance from CMS, the Department of Labor, and the Department of the Treasury, insurance providers are not required to cover testing for surveillance or employment purposes. Clinical decisions for testing are to be made by the attending health care provider and may include individuals with symptoms as well as individuals without symptoms who are known or suspected of recent exposure. Currently, many states are providing funding and other resources to support increased testing in LTC facilities and some have increased facility payment rates to help with new costs related to COVID-19. However, questions remain about the sustainability of these supports and who should pay for testing of those not covered by Medicare or Medicaid, particularly as states consider ongoing retesting of staff in these settings.
State Actions to Require or Enhance COVID-19 Testing in LTC Facilities
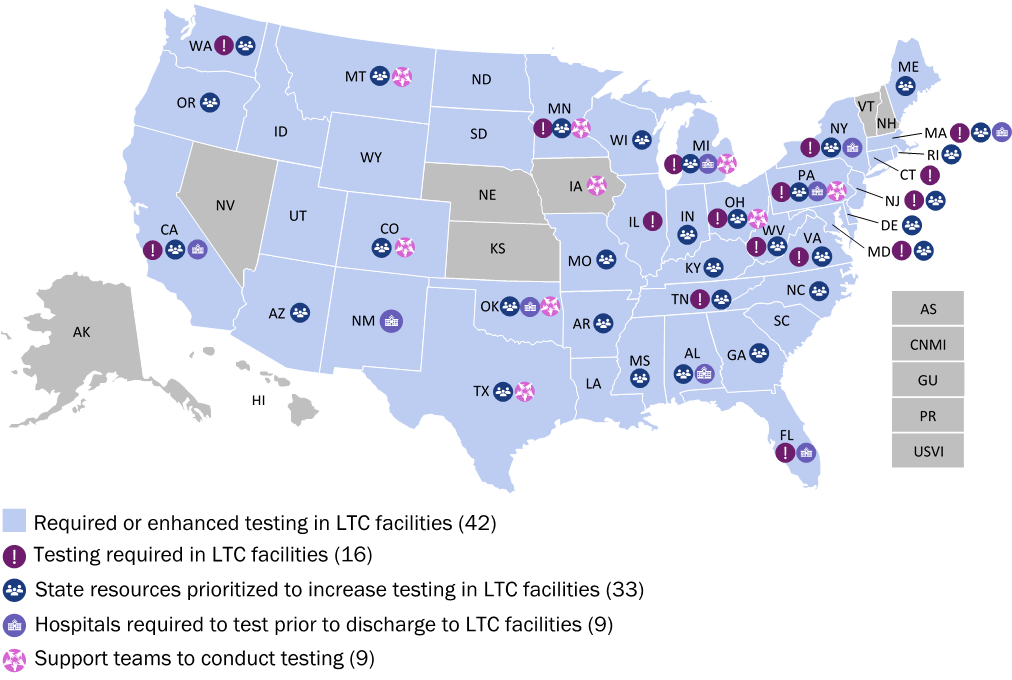
At least 42 states have taken steps to require or enhance testing of residents and staff at LTC facilities.
- At least 16 states require testing of residents, staff, or both in LTC facilities. This includes baseline or initial testing of all or a subset of facilities across a state and, in some states, retesting of residents or staff, with frequencies that range from once to twice per week. Many of these states also provide supports to facilities to execute testing, such as additional funding, test kits or other resources.
- At least 33 states have taken steps to enhance testing in LTC facilities. Specific actions include announcing and implementing plans to expand testing in facilities, increasing funding to facilities to support expanded testing, providing test kits and other supplies, or dedicating teams to conduct testing (see below).
- At least 9 states require hospitals to test patients before they are discharged to nursing homes or other LTC facilities. A number of these states do not allow discharge to nursing facilities until a patient tests negative for COVID-19, with some requiring two negative tests separated by 24 hours before discharge is allowed. Other states use test results to inform discharge and appropriate cohorting and isolation of individuals at facilities.
- At least 9 states have dedicated teams that are deployed to support testing at facilities. Teams utilize an array of professionals to support and conduct testing at facilities, including public health and clinical professionals and in some cases the National Guard.
Map of State Activity
For additional information and links to state resources on testing in LTC facilities, please refer to State Actions Addressing COVID-19 in Long-Term Care Facilities.
Given the robust and rapid nature of state response efforts, we welcome feedback on state actions that may be missing or have evolved since the release of this brief. If there are examples you believe should be added or amended, please contact Michelle LeBlanc at mleblanc@nga.org.
All NGA COVID-19 memos can be found here, or visit COVID-19: What You Need To Know for current information on actions States/Territories are taking to address the COVID-19 pandemic; as well as advocacy, policy, and guidance documents for protecting public health and the economy.












