This memo provides a high-level summary of national efforts to spur development and accelerated development processes, information on COVID-19 vaccine candidates in advanced stages of development, including where investments have been prioritized, and an appendix with information on global development efforts.
Introduction
Achieving broad immunity to COVID-19 is central to a return to normal life, and most experts maintain that this hinges on a widely available, safe, and effective vaccine. A global effort to develop and manufacture promising COVID-19 vaccine candidates is well underway with as many as 213 vaccines in development globally, at least 10 candidates in Phase 3 clinical trials, and some estimating that one or multiple vaccine candidates could be authorized for use before the end of the year.
This document provides a high-level summary of national efforts to spur development and accelerated development processes, information on COVID-19 vaccine candidates in advanced stages of development, including where investments have been prioritized, and an appendix with information on global development efforts.
National Efforts to Spur Development
On May 15, the Trump Administration announced the framework and leadership for its Operation Warp Speed (OWS) initiative, a public-private partnership to facilitate expedited development, manufacturing and distribution of COVID-19 countermeasures including vaccines. OWS is a partnership among the Department of Defense (DoD) and several agencies within the Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA). OWS also engages with numerous other federal agencies and private entities.
To achieve its aims, OWS has made significant, strategic investments to support development and enhanced manufacturing capacity for select COVID-19 vaccine candidates, including commitments to purchase hundreds of millions of doses across manufacturers. Thus far, OWS has provided funding awards to six manufacturers of COVID-19 vaccine candidates (additional details below), drawing on almost $10 billion in supplemental funding directed to support research and countermeasure development for COVID-19. OWS is also spearheading federal vaccine distribution planning in coordination with CDC and state health departments.
In collaboration with OWS and numerous federal agencies, private sector and philanthropic partners, the NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership was established to focus on building consensus on vaccine trial designs, rapid data sharing, and alignment among public and private sectors. ACTIV has provided thought leadership and assistance with development of trial design for four of the six OWS-supported candidates.
Accelerated Vaccine Development and Regulatory Processes
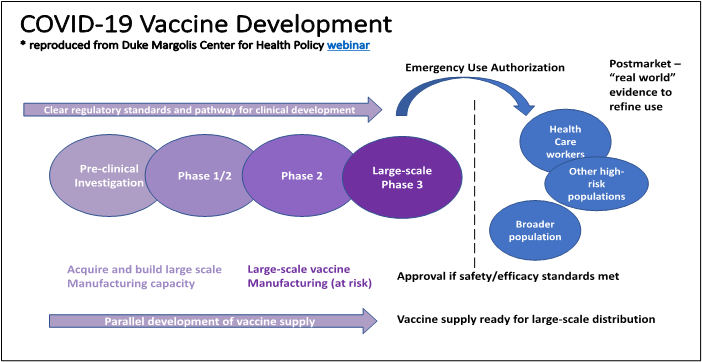
Accelerating vaccine availability is reliant upon a parallel process in which many parts of traditional development happen at once. Figure 1 below identifies the concurrent steps of clinical investigation, regulatory guidance development, and manufacturing capacity needed to expedite development. From a clinical investigation standpoint, the phases of clinical trials, are being initiated more rapidly than is typically the case during standard development (as depicted by the overlapping ovals across the clinical phases). At the same time, regulators are clarifying how they will assess vaccine candidates via written guidance, and manufacturing capacity is being built to begin production even while trials are underway. This manufacturing capacity is being built “at risk” with full knowledge that production has begun on promising candidates that may ultimately fail. These efforts are already rapidly producing hundreds of thousands of doses.
As part of this accelerated parallel process, it is anticipated that the regulatory pathway for initial authorization of COVID-19 vaccines will be an emergency use authorization (EUA). The EUA mechanism gives FDA additional flexibility to authorize the use of certain medical products in the context of an emergency. The FDA has already granted several EUAs for diagnostics, therapeutics, and personal protective equipment during the COVID-19 pandemic. Typically, a product may be considered for an EUA if it is determined that the known and potential benefits of the product outweigh the known and potential risks. However, given that vaccines are intended for widespread use in predominantly healthy individuals, the FDA has indicated that an EUA for any COVID-19 vaccine candidate will require evidence of safety and efficacy that more closely aligns with traditional vaccine approval and licensure processes. This includes demonstrating a minimum of 50 percent efficacy and at least 2 months of follow-up safety data on half of the participants enrolled in the Phase 3 trial, as published in FDA’s specific guidance for industry. The guidance aims to provide COVID-19 vaccine developers and other stakeholders with expectations for the data and information needed to support the agency’s full assessment of the risks and benefits associated with the vaccine’s safety and efficacy profile.
Additionally, the FDA notes that individual assessments will be made on a case by case basis considering the target population, the characteristics of the product, the preclinical and human clinical study data on the product, and the totality of the available scientific evidence relevant to the product. It is also expected that any manufacturer that is granted an EUA for their vaccine candidate would continue collecting safety and efficacy data to work towards full licensure and approval as soon as possible.
Several mechanisms exist to inform and advise the FDA’s review of medical products, including COVID-19 vaccines. The Vaccines and Related Biological Products Advisory Committee is a body of independent experts that publicly reviews and evaluates data concerning the safety, effectiveness, and appropriate use of vaccines and related biological products and provides recommendations to the FDA to guide authorization, approval, and regulation of these products. Following FDA authorization or approval, the CDC’s Advisory Committee on Immunization Practices (ACIP), a federal advisory committee, makes recommendations regarding the use of vaccines in various populations (e.g., specific age groups, professions or other groups at risk of infection). ACIP reviews all available safety and efficacy data of vaccine candidates to support decision-making on the benefit-risk balance for recommending use in target populations. ACIP established a COVID-19 Vaccine Workgroup in April 2020 to help inform evidence-based approaches to COVID-19 vaccination policy and provide draft recommendations to the full ACIP, using an open and transparent process for decision-making. These recommendations will relate to equitable allocation of COVID-19 vaccines among the U.S. population and specific recommendations for each COVID-19 vaccine that is authorized or approved by FDA. The full ACIP committee has met publicly each month since June to discuss COVID-19 vaccine candidates. Presentations from the August 22nd ACIP COVID-19 Vaccine Workgroup meeting can be accessed here.
COVID-19 Vaccine Candidates in Development
As of October 14, approximately 192 to 213 vaccines are in development globally for COVID-19 and at least 11 are in Phase 2/3 or Phase 3 clinical trials. Both existing and novel approaches to vaccine development are being investigated (see Table 1). [1] Novel vaccine platform technologies have potential to accelerate the pace and scale of development and production, but may require additional regulatory scrutiny given they are largely unproven modalities. COVID-19 vaccines in development also vary with regard to the diversity of populations represented in trials, dosing regimens, storage and handling requirements, and other factors that will have significant implications for how they are ultimately distributed and administered once approved. Notably, all but one vaccine candidate currently in Phase 3 trials in the United States require two-dose regimens.
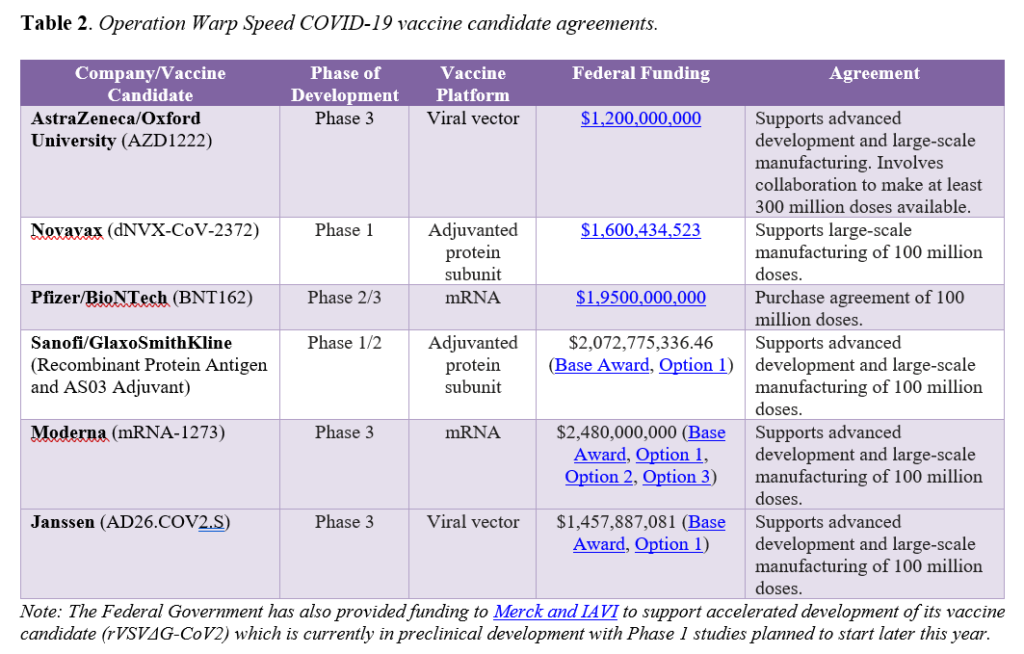
To spur development of promising vaccines for COVID-19, the Trump Administration’s OWS initiative has targeted specific candidates for investments. OWS has six agreements with select manufacturers to support development, manufacturing capacity, and/or purchase doses of vaccine candidates. Importantly, the agreements vary in scope, with some supporting development activities in addition to helping build manufacturing capacity, while others focusing solely on manufacturing. All agreements include a commitment of or intent to collaborate on a specified number of doses to be provided for distribution by the federal government (see Table 2).
Beyond those prioritized by OWS, there are at least seven other COVID-19 vaccine candidates in Phase 3 trials globally. Additionally, both China and Russia have approved COVID-19 vaccines for use in certain populations without results from Phase 3 trials, which most experts agree raises significant questions around efficacy and poses potential serious safety risks. While it is anticipated that a COVID-19 vaccine may be granted EUA in the United States by the end of the year, there are still many unknowns. Both Janssen (Johnson & Johnson) and AstraZeneca/Oxford recently paused clinical trials for their vaccine candidates to investigate adverse events that occurred. AstraZeneca/Oxford resumed clinical trials in the United States on October 23 following the resumption of trials in other countries and Janssen is preparing to resume its trial soon. Such study pauses are common during large-scale clinical trials and serve as a reminder that delays may occur and a certain time for EUA application and authorization is not guaranteed. Further, the landscape will undoubtedly evolve over the next three to six months as other candidates progress through clinical development and more is known about the safety and efficacy of the specific vaccines. As development unfolds and one or more COVID-19 vaccines are authorized or approved, new questions will emerge around the impact of authorized or approved vaccine availability on ongoing trial participation, how vaccines compare to one another, whether real-world effectiveness matches trial efficacy, and safety monitoring, among others.
For more information, please contact:
- Kirk Williamson (kwilliamson@nga.org), Policy Analyst, NGA Center for Best Practices
- Kate Johnson (kjohnson@nga.org), Program Director, NGA Center for Best Practices
Appendix: Global Collaborations to Accelerate COVID-19 Vaccine Development
The World Health Organization (WHO). The WHO is directing and coordinating international efforts to develop and evaluate vaccines by facilitating collaboration among scientists, developers, and funders; mapping candidate vaccines and their progress; producing a set of minimum desired attributes of safe and effective vaccines; and coordinating clinical trials across the world. In April, the WHO launched the Access to COVID-19 Tools (ACT) Accelerator, a global collaboration to accelerate development, production, and equitable access to COVID-19 tests, treatments, and vaccines. The vaccines pillar of ACT Accelerator, COVAX, is focused on speeding up the search for an effective vaccine for all countries, supporting manufacturing capabilities, and buying and coordinating supply so that 2 billion doses can be fairly distributed around the world by the end of 2021. COVAX is rooted in a global risk-sharing approach to vaccine development and access. Additionally, the WHO is leading a coordinated international, concurrent randomized controlled Phase 3 trial (the Solidarity Trial) of different vaccine candidates, aiming to enroll 280,000 participants from at least 34 countries. Interim results are expected early 2021.
Gavi, the Vaccine Alliance (Gavi). Gavi is an international organization established in 2000 to improve access to new and underused vaccines for children living in the world’s poorest countries. Along with the WHO and the Coalition for Epidemic Preparedness Innovations, Gavi is co-leading global COVAX efforts to accelerate development and ensure equitable global supply of COVID-19 vaccines. Specifically, Gavi is coordinating the procurement and financing mechanisms of COVAX, COVAX Facility and COVAX Advance Market Commitment (AMC). COVAX Facility pools purchasing power across self-financed countries, while COVAX AMC supports access to COVID-19 vaccines for lower-income countries through investments from sovereign donors, philanthropies, and the private sector. Combined, these efforts aim to ensure all countries can participate regardless of their ability to pay. As of late August, 80 potentially self-financing countries have submitted non-binding expressions of interest to the Gavi-coordinated COVAX Facility and 92 low- and middle-income economies are eligible to be supported by the COVAX AMC. Notably, the Trump Administration has explicitly stated that the United States will not participate in COVAX.
The Coalition for Epidemic Preparedness Innovations (CEPI). CEPI is a global partnership of public, private, philanthropic, and civil organizations formed in 2017 to develop vaccines to stop future pandemics. Specifically, CEPI advances vaccine development through proof-of-concept to human testing and establishes vaccine stockpiles in preparation for epidemics; funds new and innovative platform technologies; and coordinates activities to improve collective response to epidemics. In coordination with the WHO and Gavi, CEPI is leading COVAX vaccine research and development work, which aims to develop three safe and effective vaccines that can be made available to countries participating in the COVAX Facility. CEPI is currently supporting nine vaccine candidates and recently announced an agreement with SK bioscience, a South Korean pharmaceutical company, to reserve manufacturing capacity to produce 2 billion doses of a safe and effective vaccine by the end of 2021.
All NGA COVID-19 memos can be found here, or visit COVID-19: What You Need To Know for current information on actions States/Territories are taking to address the COVID-19 pandemic; as well as advocacy, policy, and guidance documents for protecting public health and the economy.