Background
The emergence of the COVID-19 pandemic has created unprecedented public health challenges and spurred a global race to develop and distribute one or more viable vaccines. The challenge of vaccine development is matched by the challenge of vaccine distribution; once discovered and produced, it must be delivered and dispensed to the population writ large. Although a vaccine is not yet available, lessons learned from the acquisition and distribution of COVID-19 diagnostics and therapeutics suggest that governors may want to begin addressing the challenges of mass distribution before its arrival. Immunizing the U.S. population against COVID-19 will likely require the single largest vaccination campaign ever undertaken and governors will play a key role in bringing together leaders from their state public health, immunization, and emergency management systems to design and execute the operation. As with many COVID-19 activities, a “whole of government” response, with broad participation by health and human services, economic development, education, and public safety agencies, as well as private sector partners and the public, will be critical to its success.
This memorandum provides:
- Actions for Governors Preparing for Mass Distribution of a Vaccine
- Overview of Planning Considerations for Mass Distribution
- Distribution: From Industry to States
- Distribution: From States to the Public
- Prioritization of Critical Populations
- Previous Planning Efforts and Lessons Learned
- Addressing Vaccine Hesitancy and Misinformation
- Legal Considerations
This memorandum reflects the information available as of its publication date. There is a high degree of uncertainty as to the exact processes and procedures that will be used for operations, administration, and logistics. This memorandum will be updated as major changes are announced.
Actions for Governors Preparing for Mass Distribution of a Vaccine
While the arrival of a vaccine may be months away, preparing for its eventual distribution can begin now. Governors may want to consider the following early actions:
Convene partners to begin planning. Governors can convene cabinet officials and key external stakeholders to devise a preliminary strategy, review possible scenarios, organize operations, and innovate new solutions. While public health agencies will likely take the lead on any mass dispensing effort, the sheer scope and size of a COVID-19 vaccination campaign, as well as its implications for reopening, will require partnerships from stakeholders across multiple governmental and non-governmental agencies.
Continue to advocate for federal guidance and clarification. The process by which vaccine will ordered, delivered, dispensed, and paid for remains unclear. While federal agencies have provided some detail, planners from public health, immunization, and emergency agencies have outstanding questions. States need clearer guidance from federal agencies to ensure that all partners can effectively plan for mass vaccination.
Empower staff to build out vaccine distribution scenarios. Governors can direct key staff to review potential scenarios, update mass dispensing plans, and discuss key decisions that will need to be made to acquire, prioritize, and dispense vaccines effectively. This includes clarifying roles and responsibilities, evaluating assets, and identifying gaps and opportunities. Tabletop exercises may prove a useful tool in surfacing potential challenges so that mitigations and solutions can be devised.
Begin considering how vaccines should be prioritized. Specific guidelines for the prioritization of COVID-19 vaccine are under development; however, HHS has stated that their eventual recommendations will “adjust” the guidance provided by pandemic influenza planning. Governors and their agencies can use this more general guidance to begin identifying key decisions related to vaccine prioritization, including how groups will be identified, how adjustments to prioritization will be made, and how prioritization will be communicated to the public. Further detail is provided later in this memorandum.
Assess logistical capability. As with COVID-19 testing and PPE distribution, many of the anticipated challenges with vaccine dispensing revolve around logistics. In advance of a vaccine, governors may want to work with their public health and emergency management advisors to understand existing supply chains for vaccine/immunization programs, historical strategies to distribute vaccine and anticipated capacity, and assess their state’s ability to receive, store, and distribute large amounts of vaccine. If states are able to conduct vaccination operations, they will need to anticipate associated equipment needs and supply sources. To that end, evaluating their ability to provide cold chain storage as well as supplies such as personal protective equipment (such as gloves), hypodermic needles, and disinfecting wipes will be useful in overall planning.
Determine communication strategies. Recent polling shows only half of Americans plan on getting the COVID-19 vaccine once it is made available. With vaccine coverage an essential part of population protection, governors may want to begin communicating about vaccination now. Governors can work with their agency officials, as well as community groups and private sector partners, to determine the communication strategies that will improve vaccine uptake and reduce hesitancy.
Overview of Planning Considerations for Mass Distribution
Based on lessons learned from COVID-19 diagnostic and treatment distribution approaches, once one or more vaccines for COVID-19 are available, distribution approaches will likely be phased. Many anticipate a limited initial supply, and priority populations will need to be identified through eligibility criteria. Over time, as supplies increase, distribution strategies to protect the entire population of the United States may be possible. Additional complicating factors, such as the need for multiple doses, non-interchangeable products, varying requirements for cold chain storage, and the need for socially distanced vaccination practices the utilization of off-the-shelf plans.
Mass dispensing is a major undertaking, and states will face operational challenges related to vaccine acquisition and distribution as well as administrative challenges related to funding, tracking, and prioritization. States will need to prepare for engagement and communications approaches to get stakeholder and broad community buy-in to the immunization approach, address issues related to vaccine hesitancy, and legal challenges related to liability, the scope of practice, and federal pre-emption. This memorandum explores the contours of these issues to provide a primer for advance planning and does not constitute exhaustive guidance on all policy issues.
Distribution: From Industry to States
On May 15, the Trump administration announced Operation Warp Speed (OWS) which aims to “deliver 300 million doses of a safe, effective vaccine for COVID-19 by January 2021.” OWS includes major federal investments in, and support for, the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. Once a vaccine is developed, the federal government will likely purchase and secure all available product and centralize distribution to states. The algorithms for allocation are unknown at this time, but current recommendations for vaccination apportionment suggests that it be done in proportion to state and territory populations.
With regard to distribution, OWS has stated that the U.S. Department of Defense (DOD) will assist with the distribution and administration of the vaccine. While the U.S. Department of Health and Human Services (HHS) will remain the lead agency for the federal COVID-19 response, the Defense Logistics Agency (DLA) expects to provide contract, logistics, and administrative support to the distribution process. The exact nature of how other DOD elements may be used, and how processes will be adapted, is still being defined.
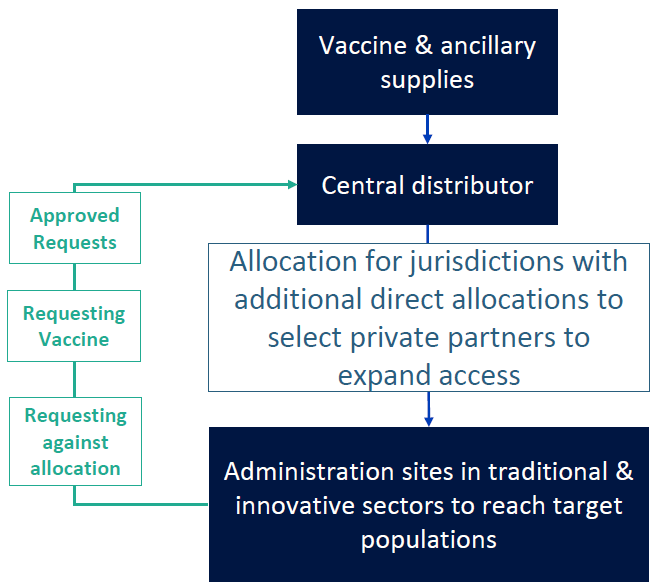
On July 30th, the CDC held a meeting with immunization stakeholders and outlined a general framework for distribution. The announcement alludes to a distribution model similar to that used for H1N1 vaccine: industry will deliver vaccine to a central distributor and states and territories will receive weekly allocations. Vaccine administration sites, including private providers, clinics, government-run points of dispensing, and others will need to make requests to the state for the vaccine, and states will prioritize and approve those requests against their daily allotment. Once a request has been approved by the state, the distribution will be made directly from the central distributor to the receiving site via contracts arranged by the DLA. Additional direct allocations will be made to select private partners (likely major retail clinics such as CVS and Walgreens) to expand access.
Distribution: From States to the Public
According to the planning guidance provided by HHS and the CDC for the 2009 H1N1 pandemic, each state, in coordination with local health departments, can recruit vaccine providers and sites to be pandemic vaccine providers. Providers willing to administer the vaccine enroll with state health departments and agree to requirements for receiving, storing, administering, and tracking vaccine administration. Depending on the governance structure of the state, enrolled providers will place orders for the pandemic vaccine with either the state or local health department’s immunization program. The CDC provides each state a daily allocation of vaccine based on population, and states can prioritize and fill orders by the state or local immunization program against those allotments. Orders are then sent to the CDC, and vaccines will be shipped directly to the provider through a centralized vaccine distributor. For some critical workforce groups, states may consider coordinating separate vaccine clinics with employers. For example, hospitals or health systems may vaccinate their own workforce.
States may also choose to administer all or some of the vaccine through state-run vaccination sites. If so, they may use emergency mass dispensing as a model for distribution, for which extensive plans have been developed for other threats, such as anthrax. States can use a variety of methods to dispense the medication; they may elect to distribute vaccines directly to residents through state-administered PODs, or they may choose to pass the vaccine (and dispensing responsibility) through to local agencies and private sector partners. Strategies for vaccine dispensing generally fall into two models: “pull” and “push.”
- Pull models allow the public to retrieve vaccines from PODs (e.g., drive-through clinics, clinics established at schools, and other areas).
- Push models require state and local officials to push the vaccine out to entities who are responsible for delivering the vaccine to specific populations.
These methods can also be used in combination. Given the disproportionate burden of COVID-19 on communities of color, the elderly, and individuals in congregate care settings, “push models” into communities that may face barriers to vaccine access will be important to support equitable distribution for those most at-risk of harmful effects from the virus. When selecting a strategy, states should consider operational capacity, the amount of vaccine available, available staff, and facility requirements for their jurisdictions. States should also coordinate with local partners, health care facilities, businesses, and other stakeholders to distribute the vaccine from the SNS to affected areas.
Prioritization of Critical Populations
Initially, the availability of a vaccine is likely to be limited, due to manufacturing constraints. State leaders will need to make difficult decisions concerning vaccine allocation and prioritization. To most effectively and equitably allocate vaccines, state leaders should develop prioritization schedules based on CDC guidelines, disease burden, and vaccine supply.
According to HHS, a tiered approach for vaccine distribution will be utilized. The methodology for the allocation of the vaccine will build upon guidance developed as part of pandemic flu planning. The method will be adjusted based on “experience during the first wave of the COVID-19 response, data on the virus and its impact on populations and the performance of each vaccine, and the needs of the essential workforce.” CDC’s established General Principles and Interim Guidance on Pandemic Vaccination provides the general framework for how a limited vaccine should be targeted, using the following objectives:
- Maintenance of homeland and national security,
- Provision of health care and community support services,
- Maintenance of critical infrastructure, and
- Protection of the general population.
For a severe pandemic, CDC uses these priorities to establish five “tiers” of vaccination prioritization:
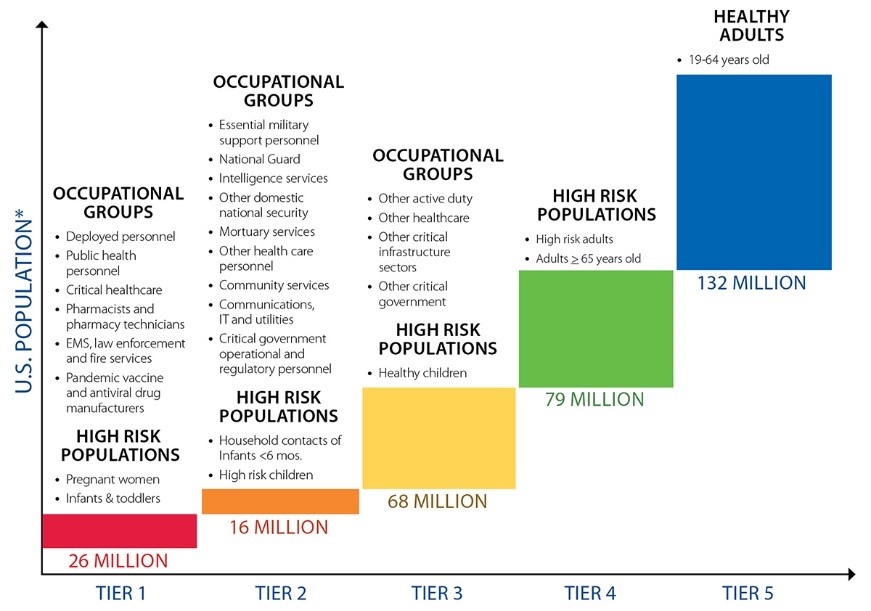
As previously mentioned, HHS will be adjusting this guidance to account for COVID-19-specific considerations. Generally, states are encouraged to follow national guidance to ensure fairness and uniformity across the U.S. and minimize confusion; however, within the parameters of the guidance, states will have the authority to distribute the vaccine to meet the specific needs of their populations. The unequal impact from the pandemic, as well as relative availability of vaccines, will cause rates of absenteeism to fluctuate between states, and the continuity of essential products and services will likely vary. Therefore, groups identified for earlier vaccination may differ between states. Additionally, state leaders, in coordination with local health authorities, will also play a role in determining when to begin offering vaccines to persons outside the initial target groups, a decision made based on local situations.
Previous Planning Efforts and Lessons Learned
States and the federal government have engaged in planning for mass distribution of pharmaceuticals before the advent of COVID-19. Every year, the seasonal influenza (flu) vaccine is manufactured and distributed for public consumption, with a national uptake rate of 45.3 percent of adults and 62.2 percent of children for the 2018-2019 season. In 2009-2010, the H1N1 influenza virus required the development and distribution of vaccines at a national scale, and state experiences during that epidemic may provide useful guidance for developing their COVID-19 vaccine strategy. Similarly, governors may consider borrowing concepts from plans developed for the distribution of Bacillus Anthracis (Anthrax) countermeasures.
H1N1 Influenza
In 2009, the H1N1 influenza uncovered operational and policy challenges across the federal government with regard to the distribution of vaccines. Similar to COVID-19, H1N1 was novel, with the majority of the U.S. population lacking immunity to the virus, and an understanding of its virulence was unknown. The H1N1 vaccine was initially available in the United States in October 2009, almost four months after the World Health Organization’s (WHO) pandemic declaration, but did not become more broadly available until late December 2009. By this time, the peak of H1N1 influenza activity had passed, and many individuals were no longer as interested in getting vaccinated. The credibility of all levels of government was diminished when the amount of vaccine available to the public in October 2009 did not meet expectations set by federal officials. Vaccine distribution was delegated to state and local jurisdictions, which were provided flexibility in deciding the best methods for distribution. For this vaccine, the federal government extended its contract with the centralized distributor responsible for public-sector purchased adult and pediatric vaccines, as well as those distributed through the Vaccines for Children Program. The distributor (McKesson) required a 100-dose minimum order and many states were forced to break down and repackage the vaccine to efficiently serve smaller vaccination sites, such as nursing homes, rural doctors’ offices, and schools. H1N1 also underscored the importance for state leaders to have well-defined, initial target groups for vaccination, and that this prioritization is communicated to both the public and public health authorities.
Bacillus Anthracis (Anthrax)
State preparedness efforts for anthrax attacks may provide additional frameworks that governors can consider leveraging in their COVID-19 vaccine planning. The mass prophylaxis protocols for anthrax provide models of government-led vaccination targeted at rapidly protecting large segments of the population. States have planned to receive countermeasures directly and then dispensed to the public, either directly to Points of Dispensing (PODs) or through intermediate receiving, staging, and storage (RSS) centers, which may be operated by state, county, or local agencies. PODs can be administered by both public and private sector agencies and may provide useful models for the distribution of vaccines.
Addressing Vaccine Hesitancy and Misinformation Although vaccines are widely understood as safe and effective public health interventions, increasing numbers of individuals are delaying or refusing vaccinations. “Vaccine hesitancy” has been used to describe a spectrum of attitudes toward vaccines ranging from skepticism to refusal and denial. While the reasons for rising levels of vaccine hesitancy are complex, experts have cited reduced fear of infectious diseases; distrust in government, science, and the medical community; the advent of “natural products”; challenges in effectively communicating scientific information to parents; and the spread of misinformation about vaccine safety. Vaccine refusal has contributed to low levels of vaccination in certain communities, leaving them vulnerable to outbreaks of vaccine-preventable diseases. In 2019, vaccine hesitancy was declared a “top threat to global health” by the World Health Organization.
The challenges of promoting vaccine confidence may be heightened in the context of a novel COVID-19 vaccine expedited through the FDA testing and approval process and deployed on a large scale. Recent polling indicates that only half of Americans reported that they intended to get a COVID-19 vaccine, with 31 percent reporting that they were not sure if they would get vaccinated and 20 percent saying that they will refuse. Likewise, numerous studies have pointed to the central role of digital and social media in organizing and advancing anti-vaccine misinformation, which is likely to accelerate as entities such as schools and health care systems move to require the vaccine. Recent research mapping pro-and anti-vaccine communities on Facebook indicates that, while anti-vaccine communities were smaller, anti-vaccine information pages were faster-growing and less insular, using campaign-style tactics to reach other communities who may be receptive to anti-vaccine narratives. The study’s authors conclude that these views may dominate in a decade absent new policy approaches to interrupt this shift toward negative views of vaccines. Anti-vaccine misinformation is also often targeted at insular communities that may have a historical distrust of government or medical institutions.
Given these dynamics, planning for mass distribution of the COVID-19 vaccine should include multifaceted communications strategies that address a range of attitudes and knowledge regarding vaccines. States should also consider strategies to proactively address vaccine hesitancy and misinformation campaigns that may fuel distrust and refusal, particularly within at-risk and underserved communities.
Legal Considerations
Governors and state leaders should not only consider the policy implications with the distribution and dispensing of a vaccine but also the legal issues that may arise in the process. To ensure rapid distribution and dispensing of a COVID-19 vaccine, NGA observes the following with respect to associated legal issues:
State emergency laws may be a helpful tool for potential expansions of existing scopes of practice for vaccine administration.
Scopes of practice set forth the range of services that licensed health care practitioners are authorized to perform. A health care professional can only provide services they are deemed eligible to perform by the terms of his/her professional license. Authority to dispense vaccines turns, at least in part, on state law, and is accomplished through several different mechanisms. While legal considerations may vary according to dispensing modality and the type of professional authorized to administer a vaccine, states can adjust vaccine dispensing through legislation, regulatory changes (e.g., health professional boards), standing orders, and emergency orders.
Federal Public Readiness and Emergency Preparedness Act (PREP) Act and state liability protections (e.g., statutes, sovereign immunity doctrines) may be helpful in addressing immunity issues related to medical countermeasure (MCM) distribution and administration.
At the state level, dispensing MCMs may benefit from leveraging relevant federal and state liability immunity laws. At the federal level, compensation programs exist to redress rare harms associated with vaccinations. There are several federal laws of import pertaining to immunity from tort liability, but one in particular stands out for MCMs: the Public Readiness and Emergency Preparedness (PREP) Act. Importantly, effective February 4, 2020, HHS Secretary Alex Azar issued a declaration pursuant to section 319F-3 of the Public Health Service Act (42 U.S.C. 247d-6d) to provide liability immunity for activities that include medical countermeasures against COVID-19. State employees, including state health, homeland, and National Guard officials involved in MCM distribution and administration may also be covered under sovereign immunity protections and emergency liability immunity protections at the state level that are designed to address malpractice and/or tort claims as well.
U.S. Food and Drug Administration (FDA)’s special legal preparedness mechanisms for emergency use of regulated medical products may articulate future COVID-19 vaccination protocols, conditions, and potential preemption of relevant state law(s).
The FDA uses numerous types of authorization mechanisms for large-scale use of drugs, devices, and biological products. Using its powers for Emergency Use Authorizations (EUAs), the FDA may provide conditions with respect to distribution and administration. Such conditions may be placed on entities that distribute or administer the product, and they stipulate how distribution and administration are to be performed by them. In addition, conditions may be placed on the categories of individuals to whom, and the circumstances under which, the product may be administered. Further, the FDA may also elect to include explicit preemptory language within an authorization of the administration and distribution of a vaccine. Such language may address potential conflicts between federal and state law (e.g., EUA issuance, implementation of emergency use authorities, reliance on any governmental pre-positioning) and the type of preemption invoked. As such, states laws may be impacted around the administration of medical products, such as informed consent laws, laws requiring Institutional Review Board approval, and laws governing the prescribing or dispensing of medical products, such as laws limiting who may prescribe or dispense medical products and under what circumstances (e.g., scope of practice).
Other groups, businesses and/or other entities may try to require future COVID-19 vaccinations for health care workers.
Such efforts may need to ensure that proper exemptions and accommodations are made that align with federal and state statutes and case law.Given that vaccine distribution may aim to ensure that front-line workers, specifically health care workers, are given priority access, legal questions may arise in states where employers mandate vaccinations for this population. While at least 30 states recommend vaccinations for health care workers, NGA is aware of only two that require vaccination: New Hampshire and Rhode Island. In the absence of a current vaccine for COVID-19, one illustrative analogy of how courts may perceive potential COVID-19 vaccination mandates from private entities for health care workers derives from themes and case law pertaining to influenza vaccinations. Litigation around workplace rules and regulations governing health care workers and vaccination mandates generally emanate from two places – employee anti-discrimination lawsuits and actions taken by the U.S. Equal Employment Opportunity Commission (EEOC), as well as exemptions under the Americans with Disabilities Act and Rehabilitation Act.[3] Additionally, state-level constitutional and statutory requirements, like state-level Religion Freedom Restoration Acts that may require religious exemptions, may be brought.
Other Key Resources
- ASTHO’s Modified Scope of Practice Used by States in the 2009 H1N1 Influenza Pandemic
- CDC’s General Principles and Interim Guidance on Pandemic Vaccination
- CDC’s Roadmap to Implementing Pandemic Influenza Vaccination of Critical Workforce
- CDC’s Point of Dispensing Standards
- HHS’ Pandemic Influenza Plan
For questions or concerns related to the contents of this memo, please contact NGA staff:
- Carl Amritt (camritt@nga.org; 202.624.5318)
- Lauren Stienstra (lstienstra@nga.org; 202.624.7872)
- Katie Greene (kgreene@nga.org; 202.624.5399)
Funding for this memo was made possible (in part) by the Centers for Disease Control and Prevention. The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services
All NGA COVID-19 memos can be found here, or visit COVID-19: What You Need To Know for current information on actions States/Territories are taking to address the COVID-19 pandemic; as well as advocacy, policy, and guidance documents for protecting public health and the economy.